28 September 2021, stored away in the Kenyan government storage facility are 795,600 Pfizer vaccines, a donation from the U.S. Government, which arrived in Kenya on 17 September 2021. According to Dr. Andrew Mulwa, the acting Director of Medical Services and Head Directorate of Preventive and Promotive Health at the Ministry of Health, the reason for the delay in distribution of the vaccines was for lack of special syringes suitable for inoculation of the Pfizer vaccine. The consignment of 2.2 million specialized syringes would arrive in Kenya, almost 3 weeks later, on 2 October 2021.
Ministry of Health says it's unable to administer 795,600 Pfizer vaccines citing lack of specific syringes.
— tv47 (@nftaIienfrens) September 28, 2021
They remain hopeful that the vaccines donor, the US, will donate the syringes as well. #TV47News pic.twitter.com/KwmWOkoFo6
But why does the Pfizer vaccine require a special syringe?
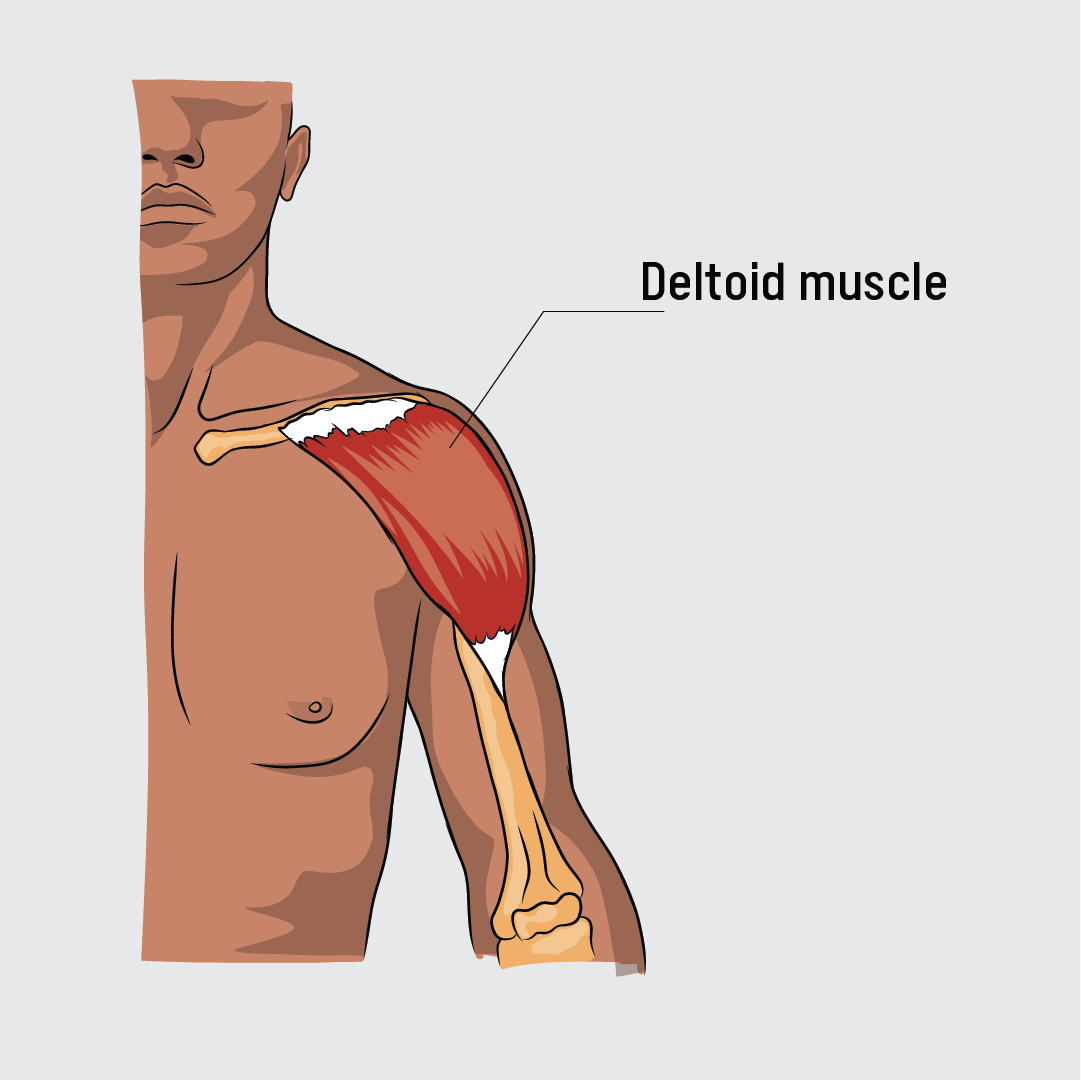
Pfizer-BioNTech (BNT162b2) COVID-19 vaccine is a messenger RNA (mRNA) vaccine developed by Pfizer and BioNTech. mRNA vaccines work by prompting the human body to make a protein that is part of the pathogen, triggering an immune response. The Pfizer vaccine is a two-dose regimen (30 μg, 0.3 ml each) given intramuscularly into the deltoid shoulder muscle at a recommended interval of 21–28 days.
In December 2020, pharmacists in hospitals across the U.S. discovered that they could extract an extra vaccine dose from the Pfizer vials that were supposed to contain only five 0.3-milliliter (ml) doses. Pfizer manufacturers lobbied for a change in the label’s formal language in fact sheets and vials provided to healthcare workers to remedy this. The change was adopted on 6 January 2021 and implemented in the fact sheets developed by the World Health Organization (WHO).
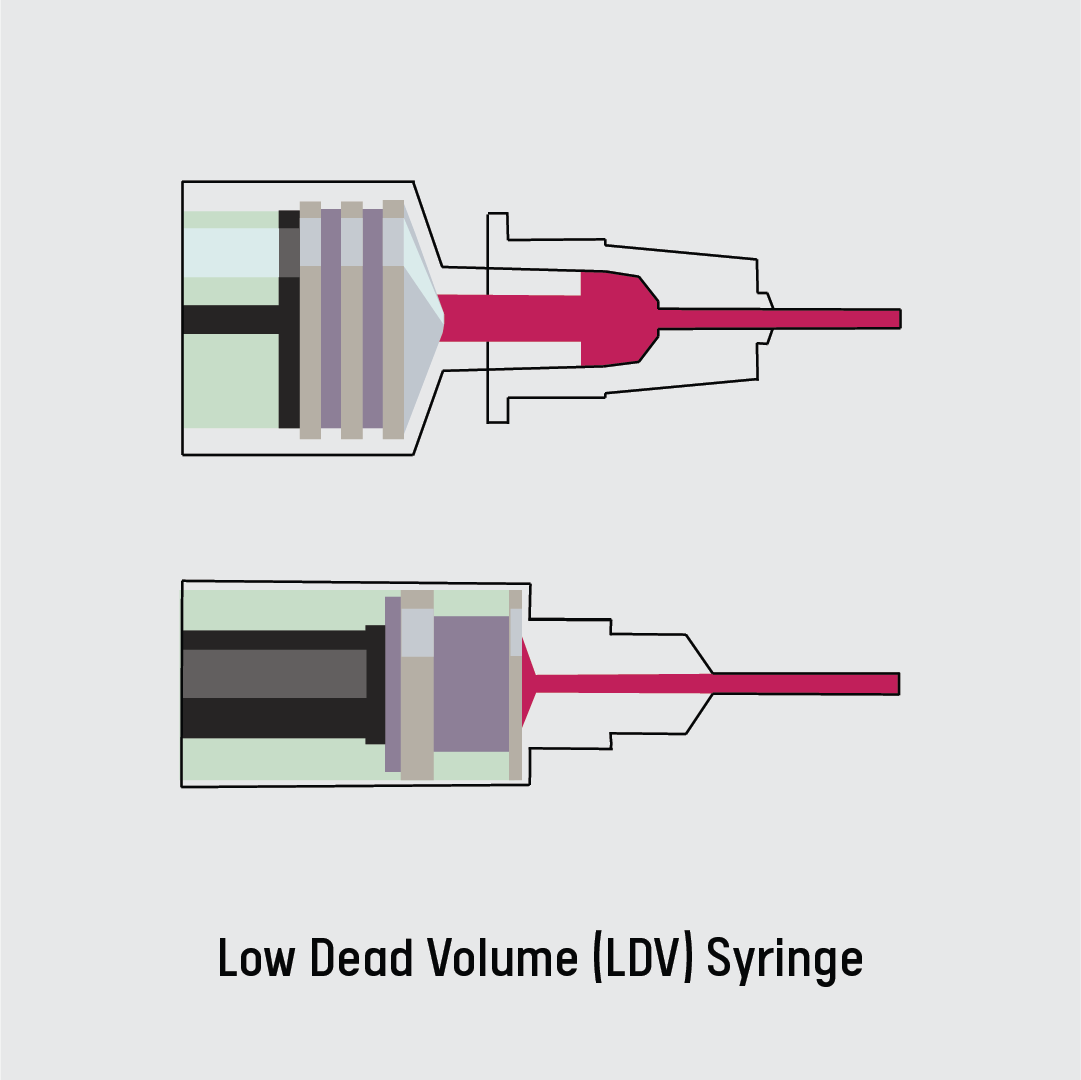
However, extracting the six doses in one vial required a low-dead volume syringe (LDV)—the Standard syringes used in Kenya draw 0.5ml. Dead volume (commonly referred to as dead space) is the volume of medical product remaining in the needle and the hub of a syringe after an injection.
Because it’s narrower, the low-dead volume syringes, which are small in size, trap less liquid between the plunger and the tip of the needle after a dose is expelled. The plungers slide all the way down to the needle to eliminate trapped liquid. Low-dead volume syringes are designed to cut down waste.
COVID-19 vaccine products do not contain any preservative and expire hours after the vial is opened. Therefore, all vaccine in a single vial must be exhausted. COVID-19 vaccines are distributed in multi-dose vials because they improve production logistics, occupy less storage space than single-dose formats, and, in most cases, sell at a lower per-dose price.
Of the five vaccines approved for use in Kenya, Janssen/J&J vials contain ten 0.5-ml doses, Moderna fifteen 0.5ml doses, and Astrazeneca two 0.5ml doses. The Sinphorma vaccine by the Beijing Institute of Biological Products, comes in a single vial of 0.5ml.
There are a limited number of manufacturers globally that produce the low-dead volume syringes. Earlier in the year, Japan had to suspend roll out of Pfizer vaccines for lack of the syringes. Some hospitals in the U.S have also complained of not getting the required syringes.
Getting to the public in a timely manner should help bring an end to the pandemic more rapidly, experts say. However, the lack of access to the LDV syringes implies that countries may have to halt vaccine-campaigns, which is unlikely, or opt for the more feasible option; fewer doses than expected.
This story was produced by Debunk Media in partnership with Code for Africa, with support from Deutsche Welle Akademie’.



